Background. Diffuse large B cell lymphoma (DLBCL) is the most common adult non-Hodgkin lymphoma. Despite therapeutic advances, 40% of the patients (pts) and especially pts older than 65 years old (y/o), will experience a relapsed or refractory (R/R) disease. Novel therapies as well as predictive biomarkers are required for these patients with a poor prognosis after standard salvage regimens. We aimed at establishing whether tumor molecular characterization by a customized target panel in pts with R/R DLBLC prior to enrollment in a phase I clinical trials might impact therapeutic decision making and outcome prediction.
Methods. Paired targeted next generation sequencing for all consecutive pts with R/R DLBCL from germline sample and fresh tumor biopsy were performed at the time of enrollment in phase I clinical trials within the early phase clinical department. Tumor and germline samples of enrolled patients were sequenced using Ion Torrent technology on an in-house customized panel of 44 genes. Molecular and cluster mapping of recurrent molecular abnormalities was determined by integrated completed likelihood (ICL) in patients with relapsed diffuse large B-cell lymphoma. Predictive and prognostic impact of molecular profile was assessed on overall survival (OS).
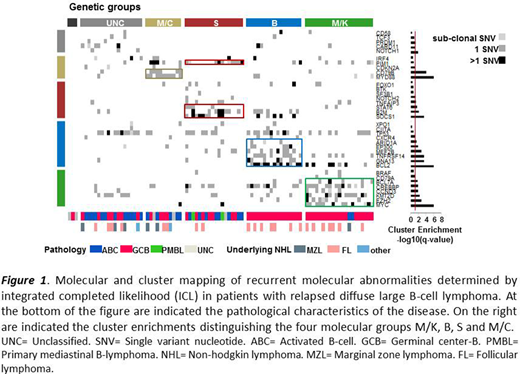
Results. Between 2013 and 2020, 89 pts with R/R DLBCL were included in the study. At time of inclusion, mean age was 62 (range 23-83) y/o, 50 pts (56%) were male, median prior lines of systemic treatment was 2 (range 1-9). Age adjusted international prognostic index (aaIPI) score was 0-1 in 42 pts (47%) and 2-3 in 47 pts (53%). Among the 82 cases with a cell of origin (COO) status assessed by immunohistochemistry, 55 (67%) pts had a germinal center (GC) and 27 pts (33%) a non-GC. The sequencing panel was informative in 86 pts (97%) and 3 pts (3%) had no variants identified. The most recurrently altered genes by mutations (m) were TP53m (n=38; 43%), CREBBPm (n=29; 33%), KMT2Dm (n=24; 27%), PIM1m (n=23; 26%) and BCL2m (n=20; 22%). Mutual exclusivity and co-occurrence analysis underlined that KMT2Dm were fully exclusive from MEF2Bm (p<0.0001); and TNFRSF14m highly significantly co-occurred with BCL2m (p=0.0001). From an unsupervised ICL clustering (based on a distance matrix derived from the presence or absence of variants within the 44 genes), 67/86 pts (78%) with one or more somatic variant could be classified into four distinct genetic groups: group M/K (n=21 patients [24%] enriched in MYCm, EZH2m and KMT2Dm; group S (n=18 patients [20%], enriched in SOCS1m, B2Mm, STAT6m and PIM1m), group B (n=17 patients [19%], enriched in BCL2m, GNA13m, TNFSRF14m and MEF2Bm) and group M/C (n=11 patients [12%], enriched in MYD88m and CD79Bm) (figure 1). Within B and M/K groups, the vast majority had a GCB COO status (n=17/17 [100%] in the B group and n=19/21 [90%] in the M/K group), whereas within M/C and S groups the COO status was evenly distributed (n=5 GC versus [vs] n=5 non-GC in M/C group; n=7 GC vs n=8 non-GC in S group). Based on their distinct patterns these four genetic groups could serve as a basis for molecular driven targeted therapeutic approaches; such as epigenetics modifiers for M/K group, JAK-STAT pathway inhibitors for S group, BCL2 and apoptosis inhibitors for B group and BTK downstream inhibitors for M/C group. As an exploratory analysis, univariate prognostic analysis for OS was performed. A shorter OS was associated with ECOG performance status ≥2 vs 0-1 (p<0.0001) and Ann Arbor stage III-IV vs I-II (p=0.0023); while the other clinical characteristics were not found significantly associated (including age ≥ 60 y/o vs <60 y/o [p=0.2741] and LDH elevated vs non-elevated [p=0.1883]). Among the most recurrently altered genes, a shorter OS was associated with GNA13m (p=0.0009), CARD11m (p=0.0147), CDKN2Am (p=0.0192) and MYCm (p=0.0220); whereas no significant association was found between the four distinct genetic patient's groups (p=0.7301).
Conclusion. A molecular tumor characterization of patients with R/R DLBCL emphasizes high incidence of TP53 and epigenetic modifying oncogenes CREBBP and KMT2D mutations. Four distinct genetic clusters were identified that could serve as the basis for a molecular-matched therapeutic approach.
Michot:Medimmune: Research Funding; Lytix Biopharma: Research Funding; Lysarc: Research Funding; Janssen: Other, Research Funding; Celgene: Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Agios: Research Funding; AZD: Honoraria, Membership on an entity's Board of Directors or advisory committees; Xencor: Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Other; Sanofi: Research Funding; Roche: Research Funding; Argen-x: Research Funding; Abbvie: Research Funding; Gustave Roussy: Honoraria, Other: Abbvie, Aduro, Agios, Amgen, Argen-x, Astex, AstraZeneca, Aveo pharmaceuticals, Bayer, Beigene, Blueprint, BMS, Boeringer Ingelheim, Celgene, Chugai, Clovis, Daiichi Sankyo, Debiopharm, Eisai, Eos, Exelixis, Forma, Gamamabs, Genentech, Gortec, GSK, H3 bio, Research Funding; Astex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Exelixis: Research Funding; Eos: Research Funding; Eisai: Research Funding; Lilly: Research Funding; Mundi Pharma: Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Amgen: Research Funding; AstraZeneca: Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Debiopharm: Research Funding; Daiichi Sankyo: Research Funding; Forma: Research Funding; Genentech: Research Funding; Kyowa: Research Funding. Varga:Astra Zeneca: Current Employment. Ribrag:Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; arGEN-X-BVBA: Research Funding; BAY1000394 studies on MCL: Patents & Royalties; Nanostring: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Immune Design: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Eisai: Honoraria; AZD: Honoraria, Other; Institut Gustave Roussy: Current Employment; Pharmamar: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; argenX: Current equity holder in publicly-traded company, Research Funding; Epizyme: Consultancy, Current equity holder in publicly-traded company, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Infinity: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal